Using the figure below, categorize electromagnetic radiation with a wavelength of 1.0 x 10-3 m. 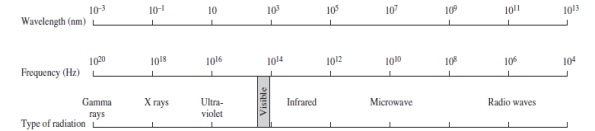
Definitions:
Periodic Table
A tabular arrangement of chemical elements organized by their atomic number, electron configuration, and recurring chemical properties.
Polar Covalent Bond
A type of chemical bond where a pair of electrons is unequally shared between two atoms, resulting in a distribution of charge.
Molecules
Collections of atoms linked in a way that forms the smallest essential part of a chemical compound capable of participating in a chemical reaction.
Carbon-Hydrogen Bond
A chemical bond between carbon and hydrogen atoms, foundational to organic molecules.
Q27: When octane (C<sub>8</sub>H<sub>18</sub>)is burned in a particular
Q29: The specific heat of gold is 0.129
Q39: Samples of the following volatile liquids are
Q51: The Lewis structure reveals an unpaired electron
Q52: Which of the following reactants can
Q58: The following data describes an initial and
Q58: The colors of the visible spectrum are
Q92: Write the Lewis structure of boron trifluoride.
Q97: What is the hybridization of the As
Q135: Identify the oxidizing agent in the