Use the Born-Haber cycle to calculate the lattice energy of MgO (s) given the following data: H(sublimation) Mg = 130 kJ/mol
I1 (Mg) = 738.1 kJ/mol
I2 (Mg) = 1450 kJ/mol
Bond energy (O=O) = 498.7 kJ/mol
EA (O) = 141 kJ/mol
EA (O-) = -780 kJ/mol
H 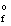
(MgO(s) ) = -601.8 kJ/mol
Definitions:
Laparoscopy
A minimally invasive surgical procedure that uses a laparoscope to view and operate on the organs inside the abdomen or pelvic area.
Culdoscopy
A diagnostic procedure that examines the female pelvic organs by inserting an endoscope through the vaginal wall.
Mammography
A specialized X-ray technique used to examine the breast for the early detection of cancer and other breast diseases.
Genitalia
The male or female reproductive organs, including both the external parts (such as the penis or vulva) and internal structures (such as the ovaries or testes).
Q8: Predict the molecular geometry and polarity of
Q12: Calculate the wavelength associated with a <sup>20</sup>Ne<sup>+</sup>
Q37: Aluminum metal has a specific heat of
Q39: If a hydrogen atom and a helium
Q41: Which of the following elements has the
Q48: What is the pressure (in atmospheres)of the
Q52: A convenient way to produce very
Q64: Chemical reactions in a bomb calorimeter occur
Q79: Which one of the following sets of
Q115: Which one of the following molecules is