Calculate the Energy Change for the Reaction K(g)+ I(g) K+(g)+ I - (G)
Given the Following Ionization Energy (IE)and
Calculate the energy change for the reaction K(g) + I(g) K+(g) + I - (g)
Given the following ionization energy (IE) and electron affinity (EA) values. 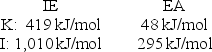
Definitions:
Modeling
The process by which individuals learn by observing the behaviors of others and the outcomes of those behaviors.
Classical Conditioning
A learning process that occurs through associations between an environmental stimulus and a naturally occurring stimulus.
Exposure and Response Prevention
A behavioral therapy technique for treating anxiety disorders that involves exposing the patient to anxiety-inducing stimuli while preventing their habitual response to the anxiety.
Benzodiazepines
A class of psychoactive drugs used primarily for treating anxiety, insomnia, and seizures by enhancing the effect of the neurotransmitter GABA in the brain.
Q13: What is the definition of a "gas"?
Q21: How much enthalpy is necessary to heat
Q32: Calculate the wavelength of the light emitted
Q47: Indicate all the types of intermolecular forces
Q49: What mass of water would need
Q55: What is the wavelength, in meters, of
Q85: What is the mass % of K<sub>2</sub>SO<sub>4</sub>
Q106: Which of the following species has the
Q108: The colors of the visible spectrum are
Q147: In the following picture, each arrow represents