Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 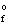 ) for LiCl(s) given the following data:
) for LiCl(s) given the following data:
H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
Definitions:
Effective Communication
The intended meaning of the source and the perceived meaning of the receiver are identical.
Efficient Communication
The ability to convey information and ideas effectively and clearly with minimal waste of time and resources.
Filtering
The intentional distortion of information to make it appear more favourable to the recipient.
Status Effects
The impacts or consequences that arise from an individual's social, professional, or other status within a group or society.
Q20: Methanol (CH<sub>3</sub>OH)burns according to the equation
Q62: Given the thermochemical equation 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q73: A face-centered cubic unit cell is the
Q76: The molecules of different samples of an
Q84: Which of the following correctly lists species
Q93: What volume of sulfur dioxide gas
Q99: For a substance that remains a gas
Q115: Oxygen gas makes up 21 % of
Q135: What is the formal charge on the
Q143: How many valence electrons does P<sup>3-</sup> have?<br>A)3<br>B)8<br>C)15<br>D)1<br>E)18