The isomerization of cyclopropane to propene follows first-order kinetics. 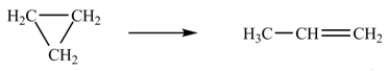 At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
Definitions:
Oxygen
A chemical element (O) vital to most life forms on Earth; it's a component of water, many organic compounds, and is required for respiration in aerobic organisms.
Photosynthetic Pathway
The series of biochemical reactions in plants and other photosynthetic organisms that capture and convert light energy into chemical energy, culminating in the production of oxygen and glucose.
Light-Dependent Reactions
The phase of photosynthesis where sunlight is captured by chlorophyll and other pigments, generating ATP and NADPH through the photolysis of water.
Carbon Fixation
Carbon fixation is the process by which inorganic carbon (typically carbon dioxide) is converted into organic compounds by living organisms, notably in photosynthesis.
Q6: Which of the following yields a buffer
Q16: Calculate the pH of a carbonated beverage
Q24: Indicate all the types of intermolecular forces
Q28: Identify the conjugate acid of HCO<sub>3</sub><sup>-</sup><br>A)H<sub>2</sub>O<br>B)CO<sub>3</sub><sup>2-</sup><br>C)H<sub>2</sub>CO<sub>3</sub><br>D)CO<sub>2</sub><br>E)H<sub>3</sub>O<sup>+</sup>
Q41: B is a catalyst in the following
Q47: Indicate all the types of intermolecular forces
Q70: Which of the following is both a
Q87: Palladium crystallizes in a face-centered cubic unit
Q89: Identify the conjugate acid of SO<sub>4</sub><sup>2-</sup><br>A)H<sub>2</sub>SO<sub>4</sub><br>B)HSO<sub>4</sub><sup>-</sup><br>C)H<sub>2</sub>SO<sub>3</sub><br>D)H<sub>3</sub>O<sup>+</sup><br>E)SO<sub>3</sub><sup>2-</sup>
Q100: An aqueous fructose solution having a density