At 700 K, the rate constant for the following reaction is 6.2 × 10-4 min-1. 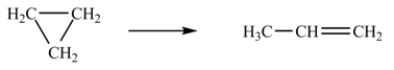 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
Definitions:
Marginal Product
The additional output gained from employing one more unit of a certain input, keeping all other inputs constant.
Labor Productivity
The measure of economic performance calculated by dividing the output by the number of labor hours used to produce it.
Technological Advance
The process of developing and applying new techniques and tools to improve efficiency, productivity, or quality in various fields.
Q16: At 340 K, K<sub>p</sub> = 69 for
Q20: Find the concentration of calcium ions in
Q22: What volume of 0.200 M potassium hydroxide
Q31: The thermal decomposition of acetaldehyde, CH<sub>3</sub>CHO
Q57: Which one of the following substances crystallizes
Q89: Which one of the following crystallizes in
Q91: Which one of these net ionic
Q95: The half life for a first order
Q100: An aqueous fructose solution having a density
Q119: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>