Consider the chemical reaction 2NH3(g) 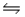
N2(g)+ 3H2(g).The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 × 10-2.Initially, 1,220 moles of NH3(g)are present.Estimate the equilibrium concentration of N2(g).
Definitions:
Specific Outcomes
Specific outcomes are the distinct and measurable results or effects that arise from implementing a certain action, policy, or strategy.
Policy Assessment Matrix
A tool used to evaluate and compare the potential effects of various policy options or interventions based on a set of criteria.
Analyzing Criteria
The process of examining the standards or conditions used for making judgments or decisions.
Continuing Research
Ongoing systematic investigation to establish facts or principles or to collect information on a subject.
Q13: What is the pH at the equivalence
Q19: What molar ratio of benzoate ion to
Q60: Calculate the molar solubility of Fe(OH)<sub>2</sub> in
Q91: What is the freezing point of a
Q91: For the reaction 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q97: An exothermic solution process is described
Q124: Calculate the pH of a solution that
Q144: What is the pH of a 0.15
Q158: What mass of sodium nitrite must be
Q171: Morphine, C<sub>17</sub>H<sub>19</sub>NO<sub>3</sub>, is often used to control