At 400ºC, Kc = 64 for the equilibrium H2(g) + I2(g) 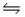 2HI(g) .If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
2HI(g) .If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
Definitions:
Cases
Instances or occurrences of a particular disease or condition being studied.
Measured Variables
Variables in an experiment or observational study that are quantified or recorded by the researcher.
Units
Basic quantities or components that serve as a standard of measurement in scientific observations or calculations.
Boxplots
A standardized way of displaying the distribution of data based on a five-number summary: minimum, first quartile, median, third quartile, and maximum.
Q26: Arrange the following aqueous solutions in order
Q32: The rate determining step must be the
Q42: Describe how to make a sodium formate
Q44: In which of the following would the
Q58: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q77: Which of the following aqueous solutions has
Q84: For the chemical reaction A
Q89: An endothermic solution process is described
Q122: A solution is 40.0% by mass benzene
Q123: The solubility of Ba(NO<sub>3</sub>)<sub>2</sub> is 130.5 g/L