For the following reaction at equilibrium, which gives a change that will shift the position of equilibrium to favor formation of more products? 2NOBr(g) 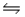
2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
Definitions:
State Tax
Taxes imposed by individual states on individuals, businesses, or property to generate revenue for state governments.
Interstate Commerce
Economic activities that cross state borders, governed by federal regulations to ensure free and fair trade among states.
Wholly-Owned Subsidiary
A company whose entire stock is held by another company, making it fully controlled by the parent company.
City Ordinance
A rule or law enacted by a local government authority to regulate, permit, or prohibit certain activities within its jurisdiction.
Q1: Arrange the following substances in order of
Q17: Arrange the following in order of increasing
Q39: Calculate the silver ion concentration in a
Q39: The reaction C<sub>4</sub>H<sub>10</sub> <span class="ql-formula"
Q64: Osmium tetroxide, OsO<sub>4</sub>, is a soft crystal
Q64: According to Molecular Orbital Theory, two
Q95: What is the mole fraction of NaOH
Q101: What is the molality of a solution
Q112: The first-order decomposition of phosphene to
Q117: How much energy (heat)is required to convert