For the following reaction at equilibrium, which change will cause the equilibrium to shift to the left? 2NOBr(g) 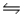
2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
Definitions:
Landscape
The visible features of an area of land, including its physical elements, living elements, and human interventions.
Freelancers
Independent professionals who work on a contractual basis rather than being employed by a single company.
Psychological Safety
The belief that one can speak up, take risks, and be creative without fear of punishment or ridicule within a group or organization.
Effective Team
A group of individuals who work together seamlessly to achieve common goals, characterized by clear communication, cooperation, and mutual respect.
Q8: The activation energy for a certain reaction
Q19: What is the H<sup>+</sup> ion concentration in
Q70: If E<sub>a</sub> for a certain biological reaction
Q100: An aqueous fructose solution having a density
Q121: The density of ice is less than
Q125: Which of the following yields a buffer
Q138: Which would be expected to be more
Q144: What is the pH of a 0.15
Q156: A 1.5 L sample of a 0.44
Q175: The pH of coffee is approximately 5.0.How