For the following reaction at equilibrium in a reaction vessel, which one of these changes would cause the Br2 concentration to increase? 2NOBr(g) 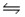
2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
Definitions:
Market Value
Market value determines an item's current selling price in the marketplace, taking into consideration supply and demand factors.
Inventory Value
The total cost or market value of all the goods and materials held by a company intended for sale.
Beginning Inventory
The total value of a company's inventory at the start of an accounting period.
Net Sales
The amount of sales generated by a company after deductions for returns, allowances for damaged or missing goods, and discounts.
Q13: A certain reaction, reaction A
Q25: For a given substance the entropy always
Q35: Aspartic acid (C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>), one of the 20
Q38: Which one of the following equations represents
Q45: Potassium crystallizes in a body-centered cubic lattice.How
Q54: Which of these acids is stronger, H<sub>3</sub>PO<sub>4</sub>
Q58: Choose the substance with the higher entropy
Q78: For the reaction whose rate law is
Q97: The vapor pressure of ethanol is 400
Q123: Which of the following is consistent