For the reaction at equilibrium: 3Fe(s) + 4H2O(g) 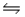 Fe3O4(s) + 4H2(g) , removing some of the product, Fe3O4(s) , would:
Fe3O4(s) + 4H2(g) , removing some of the product, Fe3O4(s) , would:
Definitions:
Health-Care Industry
Comprises sectors providing medical services, manufactures medical equipment or drugs, provides medical insurance, or otherwise facilitates the provision of healthcare to patients.
Price Elasticity
a measure of the responsiveness of demand or supply to changes in price, indicating how quantity demanded or supplied reacts to price variations.
Demand Curve
A graphical representation showing the relationship between the price of a good and the quantity demanded.
Price Elasticity
A measure of how much the quantity demanded of a good responds to a change in its price.
Q15: Hard water deposits (calcium carbonate)have built up
Q23: The entropy of any pure substance at
Q62: Find the temperature at which water boils
Q69: The molar enthalpy of vaporization of hexane
Q71: An environmental chemist obtained a 200.mL sample
Q87: The OH<sup>-</sup> concentration in a 7.5 ×
Q99: For the reaction 2NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="For
Q106: Which of the following is consistent
Q115: For the following reaction, <span
Q116: Which of the following is consistent