For the reaction at equilibrium: CO(g) + H2O(g) 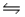 CO2(g) + H2(g) , increasing the volume of the container would:
CO2(g) + H2(g) , increasing the volume of the container would:
Definitions:
Dividends
Payments made by a corporation to its shareholder members, representing a distribution of the company's profits.
Annually
A term that pertains to an event or item that occurs once every year.
Anticipated Growth
Anticipated growth refers to the expected increase in size, value, or importance of something, such as a company's revenue or an economy's GDP, over a future period.
Estimated Dividend
The predicted amount that a company is expected to pay out in dividends to its shareholders in the near future.
Q4: A bonding molecular orbital is of lower
Q11: The pOH of a solution is 10.40.Calculate
Q13: The hydronium ion and the hydroxide ion,
Q13: What is the pH at the equivalence
Q21: Given the following information: 2A(g)+ B(g) <img
Q30: Find the temperature at which K<sub>p</sub>
Q36: For the reaction H<sub>2</sub>O<sub>2</sub>(g) <span class="ql-formula"
Q85: Given the rate law for a reaction,
Q98: A 100.mL sample of water is taken
Q161: If the pH of pure water is