The Equilibrium Constants for the Chemical Reaction N2(g)+ O2(g) Hº < 0
B)The Partial Pressure of NO(g)is Less at 2,200 K
The equilibrium constants for the chemical reaction N2(g) + O2(g) 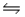
2NO(g) are KP = 1.1 × 10-3 and 3.6 × 10-3 at 2,200 K and 2,500 K, respectively.
Which one of these statements is true?
Definitions:
Liquidated Debt
A debt for which the exact monetary value has been determined and is acknowledged by all parties involved.
Unliquidated Debt
A debt or claim where the amount owed has not been determined or agreed upon by the debtor and creditor.
Different Performance
The execution of contractual obligations in a manner that varies from the exact terms agreed upon, potentially leading to disputes.
Machiavellianism
Causes someone to view and manipulate others purely for personal gain.
Q20: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q56: Which of the following statements is false?<br>A)A
Q80: Use the graph of vapor pressure to
Q81: List the following solutions in order of
Q90: Appropriate units for a second-order rate constant
Q96: The term "proof" is defined as twice
Q101: Which would have the stronger intermolecular forces
Q116: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×
Q144: What is the pH of a 0.15
Q162: When 2.0 × 10<sup>-2</sup> mole of nicotinic