If the reaction 2H2S(g) 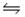
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.0120.Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g) ?
Definitions:
In Utero
A Latin term referring to the period when an embryo or fetus is inside the uterus.
Catholic
Relating to the Roman Catholic Church, its teachings, practices, and beliefs.
Yom Kippur
The holiest day in Judaism, marked by fasting and atonement, observed with intensive prayer and repentance.
Parenterally
The administration of medication or nutrition by injection through routes other than the digestive tract, such as intravenously, intramuscularly, or subcutaneously.
Q32: In the reaction: CH<sub>3</sub>COOH(aq)+ NH<sub>2</sub><sup>-</sup> (aq) <img
Q64: Osmium tetroxide, OsO<sub>4</sub>, is a soft crystal
Q71: What is the correct equilibrium constant expression
Q89: In general, to calculate the rate
Q91: Octane is a liquid component of gasoline.Given
Q92: Which of following can form hydrogen bonds
Q104: The following reaction in aqueous solution
Q109: Which of the following molecules is polar?<br>A.CH<sub>3</sub>OH<br>B.H<sub>2</sub>O<br>C.CH<sub>3</sub>OCH<sub>3</sub>
Q124: The pH of a Ba(OH)<sub>2</sub> solution is
Q128: A 8.0 M solution of formic acid