Consider the reaction N2(g)+ 3H2(g) 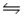
2NH3(g).If nitrogen is added to the system at equilibrium, what will happen to the ammonia concentration?
Definitions:
Industry Expansion
The process of a sector growing in size, output, or number of entities, often due to increased demand or technological advancements.
Normal Profits
The level of profit necessary to cover the costs of the inputs owned by the firm, acting as the minimum earnings to keep the firm in business.
Competitive Equilibrium
A state in a market where supply equals demand, with no external influences altering the price or quantity.
Constant-Cost Industries
Industries where the costs of production do not change as the total output increases or decreases.
Q2: A solution was prepared such that the
Q29: Calculate the molar solubility of lead (II)fluoride
Q34: Describe how to prepare 500.mL of a
Q37: For the overall chemical reaction shown below,
Q51: An example of a covalent network solid
Q81: Methane has a heat of fusion of
Q92: In the gas phase, formic acid
Q114: The reaction 2A <span class="ql-formula"
Q120: Assuming 100% dissociation, which of the following
Q131: How many moles of NaF must be