Given the following data for the reaction: A(g)+ 2B(s) 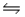
AB2(g) 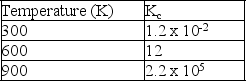 Is the reaction endothermic or exothermic?
Is the reaction endothermic or exothermic?
Definitions:
Increase Side
Refers to the side (debit or credit) of an account that is used to increase its balance, varying by account type.
Normal Balance
The side (debit or credit) on which increases are recorded in an accounting ledger; varies depending on the account type (asset, liability, equity, revenue, or expense).
Post Reference Column
A column in journal and ledger accounts that helps in cross-referencing entries between these two accounting records.
General Journal
A comprehensive record of financial transactions over the life of a company, listed in chronological order.
Q4: A 1.35 m solution of NaOCl in
Q15: A mixture made from 10 mL of
Q34: Which would have the stronger intermolecular forces
Q35: Which of the following compounds is more
Q45: When the reaction 2O<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="When
Q58: Which of the following yields an acidic
Q63: The concentration of nitrogen in water at
Q94: What volume of 0.0500 M sodium hydroxide
Q119: A city's water supply is contaminated with
Q135: Which one of these statements about strong