Consider the equilibrium C(s)+ H2O(g) 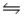
CO(g)+ H2(g), H = 2296 J What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
Definitions:
Units of X
The quantity of a particular good or service being referred to or utilized in an economic context.
Original Bundle
In economics, this refers to a specific combination of goods that a consumer initially chooses given their budget and prices.
Price Rise
An increase in the cost of goods or services, typically signifying inflation or market changes.
Budget Line
A graphical representation that shows all possible combinations of two goods that can be purchased with a given income and prices.
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q30: Indicate all the types of intermolecular forces
Q48: Calculate the pH of the solution resulting
Q51: What is the molality of an aqueous
Q64: Identify the conjugate base of HPO<sub>4</sub><sup>2-</sup><br>A)H<sub>2</sub>O<br>B)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>C)H<sub>3</sub>PO<sub>4</sub><br>D)PO<sub>4</sub><sup>3-</sup><br>E)OH<sup>-</sup>
Q68: At 10°C one volume of water dissolves
Q88: Calculate the amount of heat needed
Q106: A 100.-mL sample of water is taken
Q109: Each of the following substances is a
Q126: The meniscus for water is curved upward