Consider the equilibrium equation C(s)+ H2O(g) 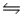
CO(g)+ H2(g), H = 2296 J.Which way will the reaction shift if the volume of the container is decreased?
Definitions:
Teenager
An individual in the age group of 13 to 19 years, typically characterized by rapid physical, emotional, and intellectual development.
Young Adult
A person in their late teens to early twenties, often considered a critical developmental stage transitioning from adolescence to full adulthood.
Bulimia
A serious eating disorder characterized by episodes of binge eating followed by purging to prevent weight gain.
Exposure
In health contexts, exposure refers to coming into contact with substances, diseases, or environmental conditions that can lead to health effects.
Q8: Which of these acids is stronger, H<sub>2</sub>SO<sub>4</sub>
Q12: The activation energy of a certain uncatalyzed
Q18: The molar solubility of lead(II)iodate in water
Q28: Arrange the following in order of increasing
Q57: Find the temperature at which K<sub>p</sub>
Q66: The thermal decomposition of acetaldehyde, CH<sub>3</sub>CHO
Q70: A sample of rainwater collected near a
Q122: Chlorine dioxide reacts in basic water
Q122: A solution is 40.0% by mass benzene
Q127: What phase exists at the point labeled