Consider the following equilibrium,
4NH3(g)+ 3O2(g) 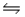
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant when the temperature is increased.
Definitions:
Safety Inventory
Extra inventory held to protect against stockouts caused by variability in demand or supply.
Supply Chain
The network of manufacturers, suppliers, and logistics providers that work together to produce and deliver products to consumers.
Trade-Offs
The balancing of factors or outcomes in decision-making, where improving one aspect may result in compromising another.
Ordering Policies
Rules or guidelines that dictate how and when to place orders for materials or products, often aiming to minimize costs and meet demand.
Q31: Which of the following aqueous solutions has
Q63: Iron crystallizes in a body-centered cubic unit.The
Q65: With respect to the system only,
Q67: The vapor pressure of a solution depends
Q79: Which of the following is consistent
Q99: At 25°C, the second-order reaction NOCl(g)
Q100: An aqueous fructose solution having a density
Q103: The solubility of gases in water always
Q121: With respect to the figure below, which
Q131: Predict the direction in which the equilibrium