Consider the following equilibrium,
4NH3(g)+ 3O2(g) 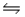
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations the reactants would increase, decrease, or remain constant after nitrogen gas was removed from the system.
Definitions:
Industrial Rock
Minerals and rocks exploited for their economic value that are not primarily sourced for their gem or precious metal content, such as limestone or sandstone.
Iron Ore
Naturally occurring rocks and minerals from which metallic iron can be economically extracted.
Gypsum
A common calcium sulfate mineral, generally formed by the evaporation of water.
Clay
A fine-grained natural soil material containing clay minerals, known for its plasticity when wet and used in ceramics.
Q6: Which of the following yields a buffer
Q37: The reaction rates of many spontaneous
Q69: The normal melting point sulfur is
Q93: The molar enthalpy of vaporization of boron
Q96: The data below refer to the following
Q101: The data below were determined for
Q104: The following reaction in aqueous solution
Q122: Write the formula for the conjugate base
Q126: Calculate the pH of a buffer solution
Q147: Calculate the pH of a 3.5 ×