The Kc for the reaction CO2(g)+ H2(g) 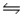
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of carbon dioxide in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Excess Reserves
Funds held by banks over and above the legally mandated reserve requirement.
Required Reserves
The minimum amount of funds that a bank is required by regulation to hold in reserve against deposits made by customers.
Total Reserves
The amount of funds that a bank or financial institution has in its vault or with the central bank, available to meet any withdrawal or payment demands.
Debit Cards
Payment cards that deduct money directly from a consumer's checking account to pay for a purchase.
Q12: Calculate the molar solubility of BaCO<sub>3</sub> in
Q21: How many grams of water are needed
Q24: What is the pH of a 0.0055
Q24: Bromothymol blue is a common acid-base indicator.It
Q37: The reaction rates of many spontaneous
Q57: Identify the conjugate base of HSO<sub>4</sub><sup> -</sup><br>A)OH<sup>-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)H<sub>2</sub>O<br>D)H<sub>2</sub>SO<sub>3</sub><br>E)SO<sub>4</sub><sup>2-</sup>
Q58: Which of the following is not true
Q90: The equilibrium constant expression for the reaction:
Q108: Which one of the following substances will
Q126: A tablet of a common over-the-counter drug