A 0.10 M NH3 solution is 1.3% ionized.Calculate the H+ ion concentration. NH3 + H2O 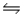
NH4+ + OH-
Definitions:
Budgeting
The process of creating a plan to spend money over a specific period, allowing individuals or businesses to determine in advance whether they can afford future activities or projects.
Selling And Administrative Expenses
Costs associated with sales and the general administration of a business, not directly tied to production.
Budgeting
Crafting a blueprint for your financial expenditure, highlighting anticipated monetary targets and the means to accomplish them.
Activity Variance
The difference between planned activity levels and actual activity levels, used in budgeting and variance analysis.
Q13: Aluminum forms a layer of aluminum
Q19: The vapor pressure of water at 20°C
Q40: At 1500°C the equilibrium constant for
Q45: Potassium crystallizes in a body-centered cubic lattice.How
Q48: A solution made of pentane and hexane
Q52: The isomerization of methyl isocyanide, CH<sub>3</sub>NC
Q61: The rate constant for a certain first-order
Q62: The compound CH<sub>3</sub>NH<sub>2</sub> reacts with water to
Q86: For water K<sub>f</sub> = 1.86°C/m.Therefore, the freezing
Q117: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img