Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3-
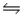
H2PO4- + HNO3 Ka(H3PO4) = 7.5 × 10-3
Definitions:
Conference Committee
A committee formed when the House and Senate need to reconcile different versions of a bill into a single version to be passed by both chambers of Congress.
House
In a governmental context, this term often refers to a legislative body or chamber, such as the House of Representatives in the United States.
Senate
The upper chamber of the legislature in various countries, including the United States, designed to deliberate and make laws.
Reelection
The act of being elected for another term in office after the completion of a current term.
Q2: What is the osmotic pressure of a
Q4: A 1.35 m solution of NaOCl in
Q9: A galvanic cell has the overall
Q13: The hydronium ion and the hydroxide ion,
Q37: A titration of an acid and base
Q50: Given the following standard reduction potentials, <img
Q52: Consider an electrochemical cell involving the
Q60: At 30°C, by how much is a
Q112: Calculate the percent ionization of formic acid
Q170: When comparing acid strength of binary acids