The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 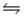
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 × 10-2 at 25°C.If Ka for formic acid (HCOOH) is 1.77 × 10-4, what is the acid dissociation constant for C7H15COOH?
Definitions:
Abusive Relationship
A relationship dynamic characterized by one party exerting power and control over the other, often involving physical, emotional, or sexual harm.
Level 3
Often refers to a third tier or stage in a hierarchical structure that could pertain to severity, complexity, or proficiency, depending on the specific context.
Marital Status
A legal or societal recognition of a person's current state regarding marriage, such as single, married, divorced, or widowed.
Personal Information
Data or details that relate to an individual's personal identity, characteristics, or life.
Q3: Write the formula of the weakest reducing
Q4: A 1.35 m solution of NaOCl in
Q29: Calculate the molar solubility of lead (II)fluoride
Q55: The following mechanism has been suggested
Q63: Appropriate units for a first-order rate constant
Q82: Write the formula for the conjugate base
Q94: Calculate the approximate freezing point of a
Q104: A 2.1 L sample of a 0.23
Q116: Calculate the cell emf for the
Q127: Using the thermodynamic data provided below, determine