Short Answer
Calculate the equilibrium constant Kc for the net reaction shown below.
AgI(s)+ 2NH3(aq) 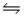
Ag(NH3)2+(aq)+ I-(aq)
For AgI, Ksp = 8.3 × 10-17; for Ag(NH3)2+, Kf = 1.5 × 107.
Definitions:
Related Questions
Q1: Which of these species has the highest
Q6: Which of the following yields a buffer
Q45: Consider the weak bases below and their
Q51: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q61: At 25 °C, the base ionization constant
Q63: Which one of these elements would give
Q72: Complete and balance the following redox
Q85: What is the missing symbol in
Q93: What mass of ammonium nitrate must be
Q101: Under what conditions (always, never, high temperature