Calculate the equilibrium constant Kc for the following overall reaction:
AgCl(s)+ 2CN-(aq) 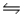
Ag(CN)2-(aq)+ Cl-(aq)
For AgCl, Ksp = 1.6 × 10-10; for Ag(CN)2-, Kf = 1.0 × 1021.
Definitions:
Dividend Payout
Dividend Payout refers to the portion of a company's earnings distributed to shareholders as dividends, representing a return on investment.
Dividend Yield
A financial measurement that illustrates the annual amount a company dispenses in dividends compared to its current stock price.
P/E Ratio
The ratio of a company's share price to its earnings per share, indicating the value the market places on its earnings.
Earnings Retention Ratio
The proportion of net earnings not paid out as dividends but retained by the company to be reinvested in its core business, or to pay debt.
Q32: Acid rain is precipitation having a pH
Q37: The reaction rates of many spontaneous
Q60: A solution was prepared such that the
Q69: The normal melting point sulfur is
Q83: The solubility of strontium carbonate is 0.0011
Q87: The OH<sup>-</sup> concentration in a 7.5 ×
Q108: Hydrogen iodide decomposes according to the equation
Q127: Which of the following is not a
Q142: The oxides CO<sub>2</sub> and SO<sub>3</sub> will form
Q158: What mass of sodium nitrite must be