Calculate the equilibrium constant for the decomposition of water 2H2O(l) 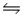
2H2(g) + O2(g)
At 25°C, given that G°f (H2O(l) ) = -237.2 kJ/mol.
Definitions:
Stockholders' Equity
The residual interest in the assets of a corporation after deducting its liabilities, representing the ownership interest of shareholders.
Equity Securities
Financial instruments representing ownership in a company, such as stocks, which provide a claim to a portion of the company's profits and assets.
Market Prices
The current value at which an asset or service can be bought or sold in a marketplace.
Carrying Value
Carrying Value, also known as book value, is the value of an asset as it appears on a balance sheet, calculated by netting the asset against its accumulated depreciation.
Q24: What is the pH of a 0.0055
Q31: Barite, anglesite, and epsomite are all examples
Q41: The Hall process involves the reduction of
Q44: Mercury, magnesium, and zinc have low enough
Q57: For the electrochemical cell Pt(s)| H<sub>2</sub>(1 atm)|
Q59: The oxidation of iodide ions by
Q61: What is the pH of 10.0 mL
Q89: The solubility product for barium sulfate is
Q108: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to sulfur
Q119: Calculate K<sub>p</sub> at 298 K for the