For the reaction CuS(s)+ H2(g) 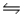
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
Definitions:
Full Court
The term used to refer to all the judges of a certain court sitting together to hear a case, as opposed to a smaller panel of judges.
Federal Appeals Courts
Courts in the United States judicial system that review decisions made by lower courts, including district courts, and are responsible for resolving appeals and legal disputes.
Lower Court Cases
Legal disputes and decisions that are handled in courts with limited jurisdiction, such as municipal or district courts.
Percent
A unit of measure used to express a proportion or fraction of 100.
Q6: Which of the following yields a buffer
Q9: Which of the following is consistent
Q15: For the nitrogen fixation reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g)
Q34: At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q39: The carbon-14 activity of some ancient Indian
Q66: The mass of a nucleus is always
Q72: HI has a normal boiling point
Q112: Write the chemical formula for the acid
Q113: What role does cadmium metal (Cd)play in
Q127: Which of the following is not a