Short Answer
For the reaction CuS(s)+ H2(g) 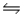
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 798 K and 1 atm pressure.
Definitions:
Related Questions
Q2: Which of these aqueous solutions has the
Q7: If the cell emf of a Zn-Cu
Q26: Write a balanced chemical equation illustrating the
Q30: Charcoal found under a stone at Stonehenge,
Q33: Based on the following options, select the
Q51: Choose the formula that does not represent
Q61: What is the pH of 10.0 mL
Q82: Write the formula for the conjugate base
Q84: At 25 °C, the base ionization constant
Q124: The pH of a Ba(OH)<sub>2</sub> solution is