Consider the reaction CO(g)+ 2H2(g) 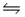
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate G° at 25°C.
Definitions:
Stigma
Group attributes that mediate a negative social evaluation of people belonging to the group.
Visibility/Concealability
Refers to how easily an aspect of a message or an object can be seen or hidden from view.
Reverse Discrimination
The practice of publicly being prejudiced in favour of a minority group in order to deflect accusations of prejudice and discrimination against that group.
Prejudiced Minority Groups
Groups that face discrimination and negative stereotypes based on characteristics such as race, ethnicity, or sexual orientation.
Q9: A galvanic cell has the overall
Q18: In [Cu(NH<sub>3</sub>)<sub>4</sub>]CO<sub>3</sub>, how many 3d electrons does
Q23: Write the chemical formula of magnetite.
Q25: The mechanism for the decomposition of
Q36: Write the formula of the strongest oxidizing
Q39: Calculate the pH of a 0.10 M
Q43: Which group of elements includes the most
Q52: Consider an electrochemical cell involving the
Q57: Find the temperature at which K<sub>p</sub>
Q77: Calculate the pH at the equivalence point