Consider the reaction CO(g)+ 2H2(g) 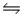
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate S°(H2(g)).
Definitions:
Undifferentiated Schizophrenic
A category of schizophrenia where symptoms do not clearly fit the criteria for other subtypes, showcasing a mix of features.
Paranoid Schizophrenic
A subtype of schizophrenia where the individual experiences delusions of persecution or grandeur, often accompanied by auditory hallucinations.
Psychotic Disorder
A mental disorder characterized by a disconnection from reality, often manifesting through delusions, hallucinations, and severely disordered thinking and behavior.
Dissociative Identity Disorder
A severe form of dissociation, a mental process which produces a lack of connection in a person’s thoughts, memory, and sense of identity, formerly known as multiple personality disorder.
Q10: Which of the following solutions is acidic?<br>A)[H<sub>3</sub>O<sup>+</sup>]
Q23: The entropy of any pure substance at
Q35: Under what conditions (always, never, high
Q52: Which one of the following compounds can
Q54: Choose the substance with the higher entropy
Q69: The normal melting point sulfur is
Q92: Calculate the pH of a 0.18 M
Q116: Calculate the cell emf for the
Q121: What pH range is a HOCl -
Q123: Which one of the following reagents is