The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) 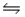 2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
Definitions:
Trace Error
A debugging process or tool used to locate and identify errors within a software program or system.
Calculate
The process of determining a result through mathematical or logical operations.
Formula
A set of symbols that express a mathematical or chemical principle.
SUBSTITUTE
A function in many programming and spreadsheet applications that replaces instances of a specified character or string within a text string with another character or string.
Q6: Which one of the following is
Q7: K<sub>p</sub> for the reaction CO<sub>2</sub>(g)+ C(s) <img
Q13: What is the pH at the equivalence
Q24: Bromothymol blue is a common acid-base indicator.It
Q40: Write the chemical equation for the reaction
Q47: Calculate the molar solubility of silver carbonate
Q48: What type of nuclear process is illustrated
Q81: The rate determining step in the following
Q96: Sodium carbonate can be made by
Q98: Beta particles are identical to<br>A)protons.<br>B)helium atoms.<br>C)hydrogen atoms.<br>D)helium