Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium sulfate is dissolved in water? 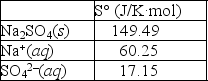 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Definitions:
New Prospects
Potential customers who have been identified as having a probable interest in a product or service but have not yet made a purchase.
Increase Sales
Strategies or actions taken to upraise the number of products or services sold, thus enhancing revenue.
Inactive Accounts
Accounts that are no longer in use or have not had any activity for a specified period of time.
Develop New Prospects
The process of identifying potential new customers who might be interested in a company's products or services.
Q1: Which of these species has the highest
Q6: Which one of the following is
Q29: Calculate the molar solubility of lead (II)fluoride
Q60: A solution was prepared such that the
Q80: For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q82: The equilibrium constant expression for the following
Q94: What volume of 0.0500 M sodium hydroxide
Q112: Write the chemical formula for the acid
Q117: Which of the following compounds is more
Q153: The overall reaction 2Co<sup>3+</sup>(aq)+ 2Cl<sup>-</sup>(aq) <span