For the reaction CuS(s)+ H2(g) 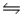
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Definitions:
Trial Balance
A spreadsheet where the totals of all ledger balances are gathered into equal totals in debit and credit columns for accounts.
Debit Column
A column in financial accounting used to record entries that decrease liabilities and increase assets or expenses.
Credit Column
A section on the right side of an accounting ledger used to record increases in liability, equity accounts, and revenue, or decreases in assets and expense accounts.
Ledger
A comprehensive collection of a company's accounts, where all transaction data from the journal is summarized and classified.
Q9: Which response has both answers correct? Will
Q20: The bond energy of F<sub>2</sub> is unusually
Q30: What mass of potassium hypochlorite must be
Q33: The CO<sub>2</sub> content of Earth's atmosphere is
Q42: In which of the following compounds is
Q49: When phosphate rock, Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s), is converted to
Q51: Calculate the pH of a 0.14 M
Q74: Determine the cell diagram for the
Q101: In the reaction CaO(s)+ SO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q121: How many <sup>14</sup>C atoms are in a