For the reaction CuS(s)+ H2(g) 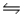
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
Definitions:
Personal Power
The influence or authority an individual has over others, derived from personal attributes or skills, rather than formal position.
Shared Leadership
A dynamic, interactive influence process through which individuals in teams lead one another.
Influence Process
The methods or techniques used to affect the behavior or attitudes of individuals or groups.
Social Exchange
Means that people build human relationships and trust through exchanges of favors based on reciprocity.
Q8: Predict the products of the electrolysis of
Q15: For the nitrogen fixation reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g)
Q34: Write a balanced chemical equation illustrating roasting.
Q39: For the reaction 2NO(g)+ O<sub>2</sub>(g) <span
Q70: Which of the following is both a
Q72: Complete and balance the following redox
Q85: In an electrolytic cell, electrical energy is
Q111: Calculate the pH of a solution that
Q117: Which of the following compounds is more
Q145: Complete and balance the following redox