The free energy of formation of nitric oxide, NO, at 1000 K (roughly the temperature in an automobile engine during ignition)is about 78 kJ/mol.Calculate the equilibrium constant Kp for the reaction N2(g)+ O2(g) 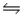
2NO(g)at this temperature.
Definitions:
Q57: Consider the following reactions and their associated
Q62: Which two compounds are produced by the
Q72: Complete and balance the following redox
Q80: A saturated sodium carbonate solution at 0°C
Q89: Identify the conjugate acid of SO<sub>4</sub><sup>2-</sup><br>A)H<sub>2</sub>SO<sub>4</sub><br>B)HSO<sub>4</sub><sup>-</sup><br>C)H<sub>2</sub>SO<sub>3</sub><br>D)H<sub>3</sub>O<sup>+</sup><br>E)SO<sub>3</sub><sup>2-</sup>
Q97: As a result of alpha emission, the
Q108: The salt hydrolysis reaction for CH<sub>3</sub>COONa that
Q111: Calculate the pH of 2.6 × 10<sup>-2</sup>
Q128: For the electrochemical cell, Fe(s)| Fe<sup>2+</sup>(aq)|| Cu<sup>2+</sup>(aq)|
Q146: Complete and balance the following redox