Consider the reaction CO(g)+ 2H2(g) 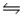
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate value of the equilibrium constant (Kp)for this reaction at 25°C.
Definitions:
Liability
Anything that is owed to other people or institutions.
Stockholders
Individuals or entities that own shares in a corporation, making them partial owners of the company and entitling them to dividends and voting rights.
Profits
The financial gain obtained when the revenues generated from business activities exceed the expenses, taxes, and costs.
Ownership of Stock
Holding shares in a company, which represents a claim on its assets and earnings.
Q15: A mixture made from 10 mL of
Q19: Using the thermodynamic data provided below, calculate
Q41: Using the thermodynamic data provided below, calculate
Q42: According to the band theory, which of
Q60: The salt hydrolysis reaction for NH<sub>4</sub>NO<sub>3</sub> that
Q64: The pH at the equivalence point of
Q70: A standard hydrogen electrode is immersed in
Q116: Which of the following does not fit
Q174: Which one of these salts will form
Q177: For maleic acid, HOOCCH=CHCOOH, K<sub>a1</sub> = 1.42