Consider the reaction CO(g)+ 2H2(g) 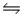
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate S°(H2(g)).
Definitions:
Market Price
The current rate at which a product or service is available for purchase or sale in an open market.
Congestion Fee
A congestion fee is a charge imposed on users of a crowded public goods, such as city roads or public transit systems, intended to reduce traffic congestion and improve air quality.
Internalize Costs
The process of ensuring that businesses or individuals take into account the external costs they impose on society in their decision-making.
Equilibrium Price
The price at which the quantity of a good or service demanded by consumers equals the quantity supplied by producers, resulting in a balance of the market.
Q1: Which of these species has the highest
Q7: Consider the following decay series: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q12: Calculate the molar solubility of BaCO<sub>3</sub> in
Q25: K<sub>w</sub> = 1.0 x 10<sup>-14</sup> at all
Q34: Write a balanced chemical equation illustrating roasting.
Q44: Which one of the following statements
Q48: Which of these metals will not be
Q55: Write the formula of the strongest reducing
Q87: Calcium carbonate decomposes at high temperatures to
Q112: Write the chemical formula for the acid