At 700 K, the equilibrium constant for the reaction CO(g)+ H2O(g) 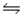
CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
Definitions:
Pew Research Center
An American think tank based in Washington, D.C., that provides information on social issues, public opinion, and demographic trends shaping the United States and the world.
Television Industry
A sector comprising companies and professionals involved in the production, distribution, and broadcasting of television content.
Ad Revenues
Money earned from selling advertising space or spots in various media formats, such as TV, radio, print, or online.
Publicity
The act of making something attract the public's attention, often through the media, to generate interest or support.
Q10: For a certain reaction, <span
Q11: Which of the following would NOT help
Q16: Consider the following decay series: <img
Q19: What molar ratio of benzoate ion to
Q28: In the complex ion [Co(en)<sub>2</sub>Br<sub>2</sub>]<sup>+</sup>, the oxidation
Q42: Describe how to make a sodium formate
Q82: You have 500.0 mL of a buffer
Q95: Which of these species would you expect
Q119: Calculate K<sub>p</sub> at 298 K for the
Q129: Calculate E°<sub>cell</sub> for the following electrochemical cell:<br>Mn(s)|