Consider the following standard reduction potentials in acid solution: 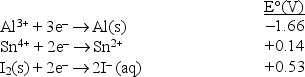 Which is the weakest oxidizing agent in this list?
Which is the weakest oxidizing agent in this list?
Definitions:
Terminally Ill Patients
Individuals diagnosed with a disease that is expected to lead to death within a relatively short time.
Family Medicine Practitioners
Medical doctors specializing in comprehensive health care for people of all ages, offering a broad array of services from preventive care to treating multiple health conditions.
Late 20th Century
The period from 1971 to 2000, marking the last three decades of the 20th century.
Deviance
Behaviors or actions that diverge from the societal norm, often eliciting negative reactions from the majority.
Q2: The first "hole" in the ozone layer
Q5: Which of the following processes would
Q9: What is the oxidation number of cobalt
Q10: Calculate the minimum concentration of Cr<sup>3+</sup> that
Q17: Select acidic oxide from the choices given.<br>A)CaO<br>B)Na<sub>2</sub>O<br>C)Na<sub>2</sub>O<sub>2</sub><br>D)P<sub>4</sub>O<sub>6</sub><br>E)KOH
Q64: When a <sup>162</sup>Re nucleus emits an alpha
Q74: Sulfur can be separated from lead
Q144: What is the pH of a 0.15
Q149: Will H<sub>2</sub>(g)form when Fe is placed in
Q177: For maleic acid, HOOCCH=CHCOOH, K<sub>a1</sub> = 1.42