Given the following standard reduction potentials, 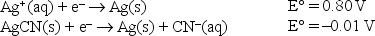 calculate the solubility product of AgCN at 25°C.
calculate the solubility product of AgCN at 25°C.
Definitions:
Adjusting Entry
Journal entries made in an accounting period's end to allocate revenues and expenses to the period in which they actually occurred.
Rent Receivable
An account in the balance sheet that represents the amount of rental payments owed to a property owner but not yet received.
Adjusting Entry
A journal entry made in the accounting records at the end of an accounting period to allocate income and expenditure to the appropriate period.
Interest Payable
A liability account on a company's balance sheet representing the amount of interest expense that has been incurred but not yet paid.
Q2: What would the atom ratio of <sup>206</sup>Pb
Q4: Alkali metal hydrides are very reactive with
Q4: Name the complex ion [Ni(CN)<sub>4</sub>]<sup>2-</sup>.
Q17: In which one of the following solutions
Q18: What element is the most abundant by
Q44: P<sub>4</sub>O<sub>6</sub> and P<sub>4</sub>O<sub>10</sub> are allotropes of phosphorus.
Q79: Al(OH)<sub>3</sub> is an amphoteric hydroxide.Write a balanced
Q94: The half-life of <sup>90</sup>Sr is 29 years.What
Q115: An electroplating solution is made up of
Q127: Calculate the pH of the solution resulting