Given the following standard reduction potentials, 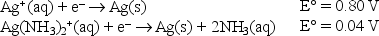 calculate the formation constant of Ag(NH3) 2+ at 25°C.
calculate the formation constant of Ag(NH3) 2+ at 25°C.
Definitions:
Moreland's Group Socialisation
A theory suggesting that individuals go through stages of membership within a group, affecting their social integration and group dynamics.
Role Transition
The process of changing from one role to another, which may involve different responsibilities, behaviors, and social expectations.
Evaluation
The systematic determination of merit, value, or significance of something, based on criteria and standards.
Group Cohesiveness
The extent to which members of a group are bonded together and commit to remaining part of the group.
Q1: A possible reaction leading to removal
Q8: Predict the products of the electrolysis of
Q59: What is the pH of a 0.20
Q67: A 0.14 M HNO<sub>2</sub> solution is 5.7%
Q92: A molecule or ion that provides an
Q99: Which one of the following combinations cannot
Q108: The salt hydrolysis reaction for CH<sub>3</sub>COONa that
Q119: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>
Q124: The radioisotope potassium-40 decays to argon-40 by
Q144: How many grams of chromium would be