Write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couple using the following standard reduction potentials in acid solution 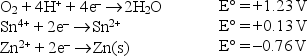
Definitions:
Racist Images
Visual representations that perpetuate stereotypes or discrimination against a race.
African American Women
Women belonging to or descending from populations primarily in the African continent, with a focus on those within the United States and their cultural and historical experiences.
Slavery
A system in which individuals are owned by others, deprived of personal freedom and forced to perform labor or services.
Femininity Stereotypes
Preconceived notions and beliefs about how women should behave and the characteristics they should possess based on their gender.
Q13: Write out the steps in the mechanism
Q26: If the measured voltage of the cell
Q27: Aluminum hydroxide, Al(OH)<sub>3</sub>, is<br>A)an acid.<br>B)an amphoteric hydroxide.<br>C)a
Q34: Write a balanced chemical equation illustrating roasting.
Q51: Using the thermodynamic data provided below, calculate
Q67: What is the coordination number of cobalt
Q75: With respect to the system only,
Q84: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="Balance the
Q89: The correct formula for the dichlorobis(ethylenediamine)chromium(III)ion is<br>A)[Cr(en)<sub>2</sub>Cl<sub>2</sub>]<sup>3+</sup>.<br>B)[Cr(en)Cl<sub>2</sub>]<sup>+</sup>.<br>C)[Cr(en)<sub>2</sub>Cl<sub>2</sub>]<sup>2+</sup>.<br>D)[Cr(en)<sub>2</sub>Cl<sub>2</sub>]<sup>+</sup>.<br>E)[Cr(en)<sub>3</sub>Cl<sub>2</sub>]<sup>+</sup>.
Q122: For which type of titration will the