Determine the equilibrium constant for the following reaction at 298 K.
2Fe3+(aq)+ H2O2(aq) 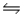
2H+(aq)+ O2(g)+ 2Fe2+(aq).
Definitions:
Distribution
The way in which values in a data set are spread or dispersed across a range.
Probabilities
A branch of mathematics dealing with the likelihood of occurrence of different events.
P-value
The probability of observing a statistic as extreme as, or more extreme than, the observed results under the null hypothesis.
Null Hypothesis
A statement asserting there is no significant difference or effect, which researchers aim to test against alternative hypotheses.
Q12: Peroxyacetyl nitrate, called PAN for short, is
Q13: Aluminum forms a layer of aluminum
Q17: Potassium superoxide, KO<sub>2</sub>(s), is used in the
Q20: Find the concentration of calcium ions in
Q35: The total number of electrons in the
Q44: Which one of the following statements
Q105: A sample of a radioisotope shows an
Q116: Calculate the cell emf for the
Q117: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q131: Determine how much energy is released when