Consider the following standard reduction potentials in acid solution: 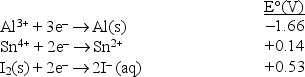 Which is the weakest oxidizing agent in this list?
Which is the weakest oxidizing agent in this list?
Definitions:
Consequences
The results or effects that naturally follow from certain actions or decisions, whether positive or negative.
Self-confidence
The belief in one’s abilities, qualities, and judgment, often contributing to one’s sense of competence and assertiveness.
Motivation Levels
The intensity of an individual's desire or willingness to accomplish tasks and achieve objectives, which can significantly influence their performance and productivity.
Subordinates
Employees or individuals who are lower in rank or position and are under the direction of a superior or manager.
Q8: Which of these acids is stronger, H<sub>2</sub>SO<sub>4</sub>
Q8: Predict the products of the electrolysis of
Q10: What happens to nitrogen dioxide in sunlight?
Q19: Using the thermodynamic data provided below, calculate
Q44: Which of these metals will reduce water
Q62: Will H<sub>2</sub>(g)form when Cu is placed in
Q93: Calculate the binding energy per nucleon of
Q99: Consider the following decay series: <img
Q122: The following nuclear equation is correctly balanced.
Q130: Ammonium chloride solutions are slightly acidic, so