Determine the equilibrium constant for the following reaction at 298 K.
2Fe3+(aq)+ H2O2(aq) 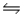
2H+(aq)+ O2(g)+ 2Fe2+(aq).
Definitions:
Schedule E
A tax document utilized to declare earnings and deficits from real estate rentals, royalty payments, partnerships, S corporations, estates, and trusts.
Schedule D
A form used with IRS tax returns to report capital gains and losses from transactions of capital assets.
Schedule K-1
A tax document issued for an investment in partnership entities, used to report the income, losses, and dividends of partners.
1099-MISC
An Internal Revenue Service (IRS) tax form used in the U.S. to report payments made in the course of a trade or business to individuals who are not employees.
Q13: Which of these ions is most likely
Q24: Which one of the following functional groups
Q26: Given the following data, estimate the
Q40: Consider the following decay series: <img
Q41: Oxidation of the 2-propanol will produce a/an<br>A)aldehyde.<br>B)amine.<br>C)alkene.<br>D)ketone.<br>E)carboxylic
Q56: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q57: For PbCl<sub>2</sub> (K<sub>sp</sub> = 2.4 × 10<sup>-4</sup>),
Q65: Methyl red is a common acid-base indicator.It
Q113: What role does cadmium metal (Cd)play in
Q144: How many grams of chromium would be