For the reaction 3Fe(s) + C(s) 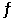 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Definitions:
Written Contracts
Formal agreements documented in writing that outline the terms, conditions, rights, and obligations of the parties involved.
Planning Process
A systematic series of actions directed towards the achievement of a goal or strategy.
Planned Change Process
A deliberate and structured approach to modify or improve existing conditions within an organization or community.
Formalizing A Contract
The process of establishing a contract in a specified, official format that is legally binding and enforceable.
Q3: When you want the application to end
Q9: Based on the following options, select the
Q19: The heaviest known isotope of hydrogen is
Q24: Carbon-11 is a radioactive isotope of carbon.Its
Q24: In order for a gas to be
Q38: A(n)_ is a block of code that
Q40: Two well-known complex ions containing Ni
Q42: Excluding water vapor, list the four most
Q53: Most invalid data is a result of
Q55: In the production of potassium metal, the