For the reaction 3Fe(s) + C(s) 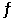 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Definitions:
Bytes
A unit of digital information in computing and telecommunications that most commonly consists of eight bits, representing a binary number.
LAN
Local Area Network refers to a computer network that interconnects computers within a limited area such as a residence, school, or office building.
WAN
Wide Area Network, a telecommunications network that extends over a large geographical distance, used to connect different local area networks (LANs) to allow for communication between them.
Ethernet
A family of technologies for local area networks (LANs) that facilitates the interconnection of computers and devices.
Q5: The equilibrium 3Fe(s)+ C(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q8: Cysteine and methionine are unique among the
Q29: Write out the reaction that shows how
Q31: Data types and classes are not related
Q39: Which statement about ozone and the ozone
Q58: Which of the following is true concerning
Q60: Three means used to concentrate ores are
Q65: Given the following notation for an
Q108: The normal freezing point of ammonia
Q114: A plot of the number of neutrons