Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 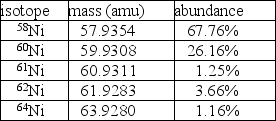 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Definitions:
Occupations
Various forms of employment or professions individuals engage in to earn a living.
Networks
Sets of nodes or points connected by relationships or pathways, often used to describe social connections, computer systems, or other interconnected structures.
Social Contacts
Social contacts include the interactions and connections one has with other individuals, forming the basis for social relationships, development, and integration into social networks.
Triadic Transformation
A concept in sociology that describes a process of change within a social system that involves three distinct stages or components.
Q25: Which of the following statements will calculate
Q30: A gas-filled balloon with a volume of
Q36: The oxidation number of Mn in KMnO<sub>4</sub>
Q74: Batteries in our cars generate electricity
Q84: A ground-state atom of manganese has _
Q84: Balance the following chemical equation:<br>C +
Q98: A soft drink costs 75 cents for
Q110: The heat of solution of calcium chloride
Q127: Define mixture.
Q132: Identify the major ionic species present in